veriseq nipt v2
Ad NGS Drives Fast Accurate And Comprehensive Detection Of Genetic Variants. Outcomes For Future Family Planning With Labs Implementing Reproductive Genetic Testing.

Veriseq Nipt Solution V2 Support
The VeriSeq NIPT Microlab STAR system screens for specific fetal chromosome abnormalities using maternal blood drawn as early as 10 weeks gestation.

. View Options VeriSeq NIPT Solution v2 Consumables Equipment List Consumables and equipment list required for the VeriSeq NIPT Solution v2. VeriSeq NIPT Solution v2 scales according to your labs needs through customized menu selection for each individual sample and versatile batch options1 With a long-lasting partnership committed to your labs growth and continued success together we can shape the future of prenatal testing. It still offers an automated next-generation sequencing-based workflow that can process up to 96 samples in.
VeriSeq NIPT Solution v2 Sample Prep Checklist Checklist for processing samples with the VeriSeq NIPT Solution v2. VeriSeq NIPT Solution v2 provides accurate information about fetal chromosomal status as early as 10 weeks of gestation using a single maternal blood draw. This product must not be used as the sole basis for diagnosis or other pregnancy management decisions.
The VeriSeq NIPT Solution v2 is an in vitrodiagnostic test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation. All workflow steps including plasma isolation cfDNA extraction library preparation and quantification and pooling and normalization are fully automated and vendor-qualified for robust. Most comprehensive view of genome-wide fetal chromosomal anomalies - IVD in-lab NIPT solution offers the broadest test.
VeriSeq NIPT Solution v2 Aneuploidii plodu pro chromozomy 21 18 13 X a Y lze detekovat s vysokým stupněm přesnosti neinvazivním prenatálním testováním NIPT které využívá celogenomové sekvenování mimo buněčné DNA cfDNA získané z krevní plazmy matky v. Equipment Height Width Depth Weight VeriSeqOnsiteServerv2 438 cm 173 in 178 cm 7in 635 cm 25 in 259kg 57lbs VeriSeqNIPT MicrolabSTARwithAutoload 903 cm 356 in 199 cm 783 in 1006 cm 396 in 160kg 353lbs VeriSeqOnsiteServerv2PlacementRequirements PositiontheVeriSeqOnsiteServerv2toallowfor. LANSM a été informée de la mise en œuvre du retrait de.
Contents Storage Requirements for the VeriSeq NIPT Solution v2. The CE-IVD VeriSeq NIPT Solution v2 is currently registered for use in Thailand Vietnam Singapore South Korea Australia New Zealand Israel South Africa and across most countries in Europe. VeriSeq NIPT Solution v2 Package Insert 200006957 v00 for Canada PDF 1 MB Aug 13 2021 VeriSeq NIPT Solution v2 Package Insert Translated into Brazilian Portuguese PDF 1 MB Aug 16 2021 VeriSeq NIPT Solution v2 Package Insert Translated into Bulgarian PDF 1 MB Aug 16 2021 VeriSeq NIPT Solution v2 Package Insert Translated into Croatian.
Reference updates do not change the software and the software instance remains unchanged. Follow the instructions provided in the Assign 20 software guide to update the references in Assign 20. Like its predecessor the VeriSeq NIPT solution v2 provides information about trisomy 21 13 and 18 as well as some sex chromosome aneuploidy.
VeriSeq NIPT Solution v2 uses whole-genome sequencing to detect partial duplications and deletions for all autosomes and aneuploidy status for all chromosomes. Trisomy 21 18 and 13 Rare autosomal aneuploidies RAAs Sex chromosome aneuploidies Partial. Outcomes For Future Family Planning With Labs Implementing Reproductive Genetic Testing.
View Options VeriSeq NIPT Solution v2. The assay provides information about fetal chromosomal status as early as 10 weeks of gestation using a single. Ad NGS Drives Fast Accurate And Comprehensive Detection Of Genetic Variants.
Clinical Trial Notification CTN identification number ID. VeriSeq NIPT v2 launched in June and took about a year and a half to develop Patel said. The new version expands the range of chromosomal and sub-chromosomal conditions associated with birth defects that laboratories can screen for.
VeriSeq NIPT Solution v2 chỉ chấp nhận số chữ cái dấu gạch dưới và dấu gạch ngang cho tất cả các trường dữ liệu. The VeriSeq NIPT Solution v2 assay enables accurate identification of fetal aneuploidy allowing detection of genome-wide fetal chromosomal anomalies with high clinical sensitivities and specificities and a low assay failure rate. VeriSeq NIPT Solution v2 Sample Prep Checklist Checklist for processing samples with the VeriSeq NIPT Solution v2.
VeriSeq NIPT Solution v2 makes NGS-based noninvasive prenatal testing accessible to any lab providing. In September last year Illumina agreed to acquire US-based healthcare company GRAIL for cash and stock consideration of 8bn. ID đợt dài hơn 26 ký tự.
View Options VeriSeq NIPT Solution v2 Consumables Equipment List Consumables and equipment list required for the VeriSeq NIPT Solution v2. Réactif VeriSeq NIPT Solution v2 - Illumina RAPPEL DE PRODUIT - Dispositifs médicaux de diagnostic in vitro - PUBLIÉ LE 20072021 Cette action de sécurité est enregistrée à lANSM sous le n R2114036. 9750 Hear from our customers what value Illuminas VeriSeqNIPT Solution v2 brings to their lab 1310 views Jul 9 2020 Watch the video to find out why laboratories In Europe have implemented.
They are available from the TruSight HLA v2 Support web page. EM0051 The Batch ID is greater than 26 characters in length. Đổi tên đợt thành tên không chứa ký tự văn bản đặc biệt nào.
VeriSeq NIPT Sample Prep Kit 24 Sample 20025895 VeriSeq NIPT Library Prep Box 24 20026030 A162813-2 A164108-2 A163501-2 VeriSeq NIPT Sample Prep Kit 48 Sample 15066801 VeriSeq NIPT Library Prep Box 48 15066809 A162738-2 A163577-2 Veuillez consulter en Annexe les numéros de codes-barres des plaques dadaptateurs dindex impactées. Unparalleled performance- Superior accuracy fastest results low failure. Documentation product files FAQs and other support resources for the VeriSeq NIPT Solution v2 VeriSeq NIPT Solution v2 Products Learn Company Support Recommended Links.
The test offers an option to request the reporting of sex chromosome aneuploidy SCA. These updates are not performed automatically and can be installed at your convenience. This noninvasive test provides an option to screen for aneuploidy in all autosomes chromosomes X Y and partial deletions and duplications greater than 7 Mb across the genome.
Illumina has launched the VeriSeq NIPT Solution v2 a CE-IVD next-generation sequencing-based approach to noninvasive prenatal testing.

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
Illumina 簇生成和测序试剂 Fc 126 1003illumina Fc 126 1003 上海易汇生物科技有限公司

Veriseq Nipt Solution V2 Genetica

Analysis Of Cfdna Using A Modified Illumina Veriseq Non Invasive Download Scientific Diagram
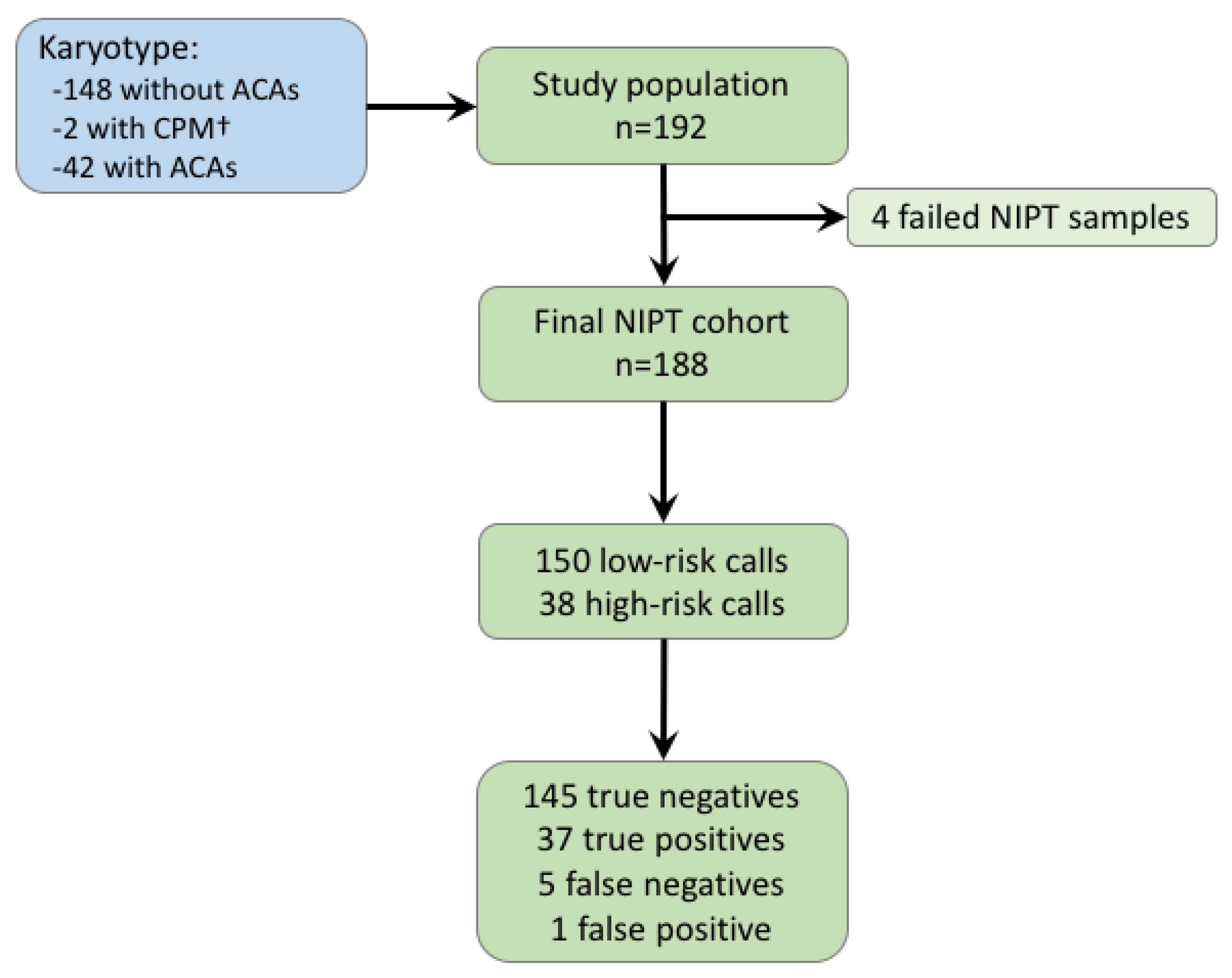
Jcm Free Full Text Strategy For Use Of Genome Wide Non Invasive Prenatal Testing For Rare Autosomal Aneuploidies And Unbalanced Structural Chromosomal Anomalies Html

Illumina Twitter પર Fdesouza Version 2 Of Veriseq Nipt Will Ship In 1h 2019 Adding Karyotype Resolution Across The Genome And Increasing The Number Of Genetic Diseases That Can Be Detected Jpm19
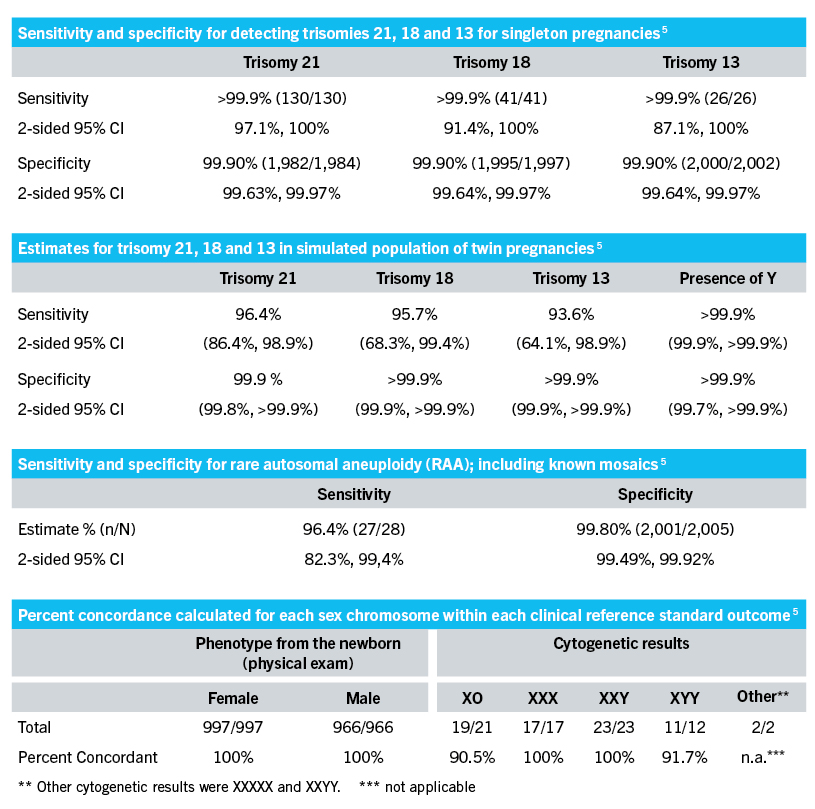
Performance Qualification Praenatest

In Vitro Diagnostic Ivd Products

Comparison Of Aneuploidy Incidence Between Study Cohorts Download Table

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution
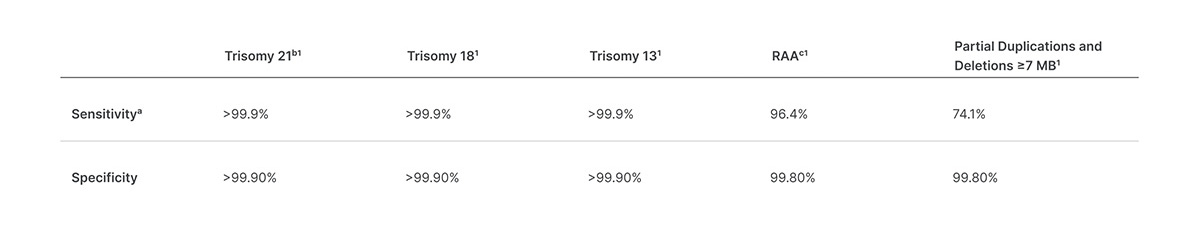
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

The Veriseq Nipt Solution Youtube

Illumina S Noninvasive Prenatal Screening Kit Receives Regulatory Approval In S Korea Business Wire

In Lab Screening With Nipt Turnkey Sample To Results In Your Lab
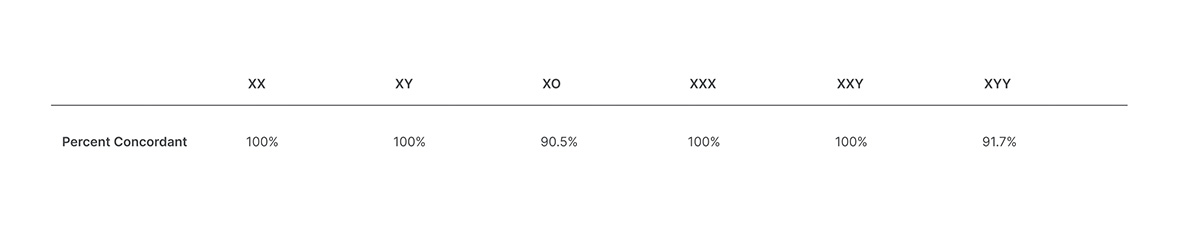
Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Veriseq Nipt Solution V2 Comprehensive And Reliable Nipt Solution

Comments
Post a Comment